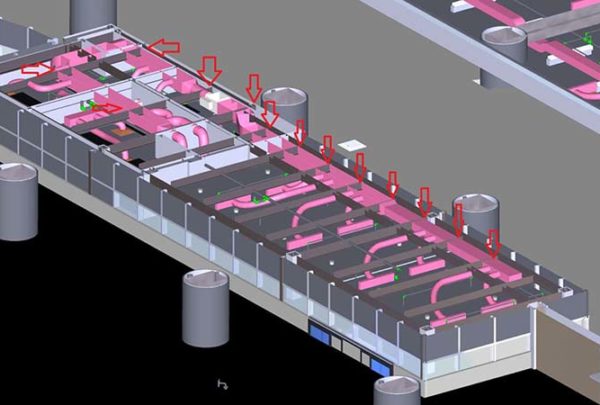
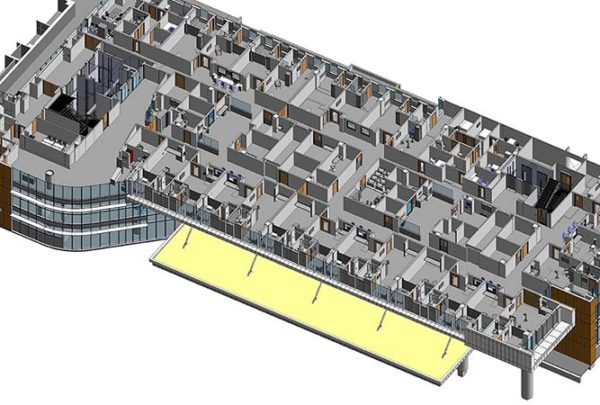
Pharmacy requirements are evolving, and requirements for the physical spaces that pharmacies occupy have tightened and become more specific, resulting in heating, ventilation and air conditioning (HVAC) challenges.
USP 800, which became effective in late 2019, sets out expectations for the safe handling of hazardous drugs in pharmacies, involving not only tight pressure requirements and high air volumes but also comfortable temperatures and a specific ceiling on relative humidity. Upgrading the pharmacy space in healthcare facilities can be a difficult and costly undertaking, and a holistic and systematic approach best ensures the best outcomes.
Pharmacies using building HVAC systems serving other hospital areas are seldom, if ever, able to meet space requirements imposed by pharmacy regulations. Pharmacy spaces have high air-exchange rates, usually above 30, so the temperature of the air to the space is not required to be below 52° to 55° Fahrenheit. The issue with using building systems is meeting the requirement of maximum of 60% relative humidity, which is attainable with space temperatures above 68°. These relative humidity levels rise as temperatures in the space are lowered, resulting in levels above acceptable ranges.
Costs to properly address evolving regulations are difficult to estimate, and disruption to existing operations can be frustrating. Understanding all the project requirements is the first step to upgrading your pharmacy correctly the first time. Considerations include:
- The location of components, such as refrigerators, allows for proper location of low sidewall inlets.
- Current and future requirements for hoods inform location for air distribution, identify the amount of air conditioning needed to cool heat loads and establish the location and quantity of power and data needed.
- Fixed size of the space allows accurate calculation of minimum airflows required to meet USP requirements for the type of space.
- Design of the space requires sealed surfaces that leave no cracks or crevices.
- Terminal clean involves surfaces able to withstand cleaning products and moisture.
- Pressure differentials required by USP allow for only small variations, with high and low settings defined by the type of space.
Equipped to meet USP 797 and USP 800 Requirements
User meetings with all involved parties at the project site are one way to understand the project requirements. A representative of the contractor, design team, consultant team, building operation team, pharmacy team and pharmacy consultant team should meet at the project location, with other necessary individuals connected virtually. The full team can then talk through the project, including disruptions to operations and other site-specific needs.
Bringing all participants together to fully understand the capital requirements, equipment needs, roadblocks and challenges is a must. A team willing to explore possibilities in addressing environmental conditions, operational challenges, and aggravation with the end in mind will solve the most complicated challenges.
Planning for a location to prepare drugs while construction is underway is a major challenge. The team must decide what they need above and beyond regulatory requirements. With the adoption of USP 800 and the requirement for a double layer of protective clothing, what temperature meets the regulatory requirement, is comfortable, and results in the correct relative humidity? The only USP requirement is that the temperature be below 68° Fahrenheit (F), but for most pharmacy projects, the temperature has been somewhere in the range of 62° to 65° F; setting a temperature below the maximum provides a cushion if the air temperature fluctuates.
Acceptable relative humidity ranges should be determined by the pharmacy team. USP standards require maintaining relative humidity below 60%. Until a few years ago, general hospital areas and systems had an operating humidity expectation of 30% to 60%. The lower range has been dropped to 20% for most areas. Some articles have suggested a lower limit for pharmacy spaces should be 40%. As you can see, humidity ranges are not standardized.
USP 797, USP 800 Space and Location Guidelines
Final design can begin after project parameters have been established. Pharmacy equipment has different requirements than that in most other areas. Cleanroom light fixtures are selected, hand-wash facilities with eyewash provisions are provided, and floors are marked to delineate clean and dirty areas. Other details that need to be addressed are:
- Location of pressure monitors to alarm out-of-parameter room conditions and for daily recording of pharmacy space conditions
- Welded seam floors and bases detailed
- Washable and cleanable ceiling tiles
- Locations of low sidewall inlets
- Selection of fan/filter units to meet filtration and air exchange requirements
Air conditioning equipment must meet the space conditions and is limited by the low temperatures and relative humidity requirements and the small air volumes of the systems. Typical hospital chilled water systems will not meet the discharge temperature requirements caused by the humidity requirements. Many pharmacy locations are in the core of the hospital without accessible roof spaces, and roof space must be nearby to use packaged rooftop equipment. Inline split system equipment with air provided from the building system is a solution if space for the equipment can be located above the ceiling or in a nearby room.
The selected equipment must produce cooling-coil air temperatures of 40° to 45° Ffor a 60° to 62° F pharmacy space temperature with relative humidity below the required maximum of 60%. These systems must cool the air continuously with a discharge air controller. Using a space thermostat to cycle equipment will not maintain the humidity level required. This equipment results in high energy use, but a system with a reclaim coil as a first stage of reheat will aid in minimizing energy use. Minimum humidity levels, although not part of current standards, should also be established. Selection of humidifiers, if needed, requires knowledge of expected low air-moisture levels and the minimum pharmacy-defined humidity level.
Selecting equipment that meets a pharmacy’s known and expected space conditions will result in a safe and regulatory-compliant space. This space will exceed required air exchanges, meet or exceed air purity requirements, aid staff in monitoring space conditions, meet anticipated future pharmacy requirements, and provide safe, reliable drug preparation areas that minimize the risk of contamination.
Operating and capital costs are a major concern for bringing pharmacy spaces into compliance with current USP 797 and USP 800 requirements. Upgrades to these spaces are not optional. Planning and properly funding an upgrade will minimize issues with future upgrades. Relocation and using temporary equipment are costly disruptions for pharmacies; proper funding will not stop the disruption now but will minimize future ones. Choosing a minimalist approach on a pharmacy upgrade puts in motion the real possibility of another upgrade when the next regulatory change is issued.
Planning and executing pharmacy upgrade projects with all affected parties involved will provide the best solution now and in the future.
Contact Haskell’s Healthcare Consulting team to ensure that your pharmacy upgrade meets your facility’s current and future needs with as little cost and disruption as possible.