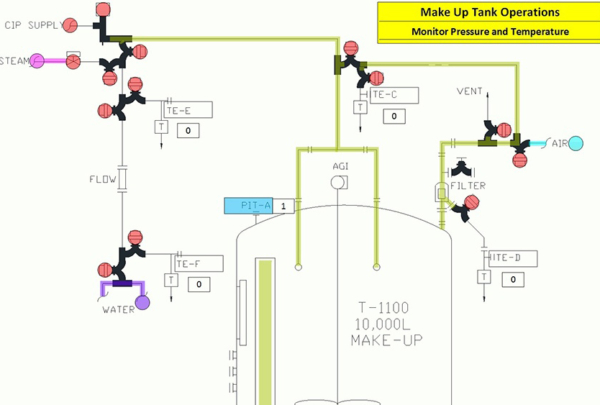
Steam-in-Place (SIP) Operations, often used in the food and life sciences industries, use the thermal energy of condensing steam for a controlled time period to affect a bioburden reduction
SIP Operations may be used for varying purposes, including the following:
- Process disinfection to kill pathogenic (disease-producing) organisms
- The thermal inactivation of a target organism
- Steam sanitization to achieve a 99.9% bio-kill of all present organisms
The most stringent SIP application achieves sterile conditions throughout the entire equipment set with the goal of sterilizing the process system as an entity. The process system may include processing vessels and piping, filtered gas overlay supplies, process liquid filters, valves, pumps and processing instrumentation.
Haskell’s process engineering team has prepared a list of 10 effective rules to help you reliably achieve sterile conditions when SIP’ing a process equipment set. Enjoy!
1. Perform Clean-In-Place (CIP) Operations Before SIP Operations
Perform effective CIP operations prior to initiating SIP operations to remove processing soils. The thermal resistance of microorganisms and spores can be enhanced by the protective effects of process soil.
2. Confirm Saturated Steam Supply
Operations should confirm steam supply header pressure and temperature to ensure a saturated steam supply. Superheated steam or an insufficient steam supply will result in a failed SIP program. Steam quality in the food industry must meet “Culinary Steam” requirements and in the biotech industry, specifications for “Clean Steam” must be met.
3. Introduce Saturated Steam Supply as High as Possible
The SIP operations should be configured to introduce the saturated steam supply as high as possible in the equipment set, leveraging the steam supply pressure and temperature differences to push out ambient system gases through monitored process low points. The ambient gases will sink to process low points for effective evacuation.
4. Install Low Point Trap Blocking Valves and Temperature Sensors
Install steam trap blocking valves and a steam trap at each process low point and include a temperature monitoring device between the valve and steam trap to monitor temperature at each process low point. Be sure to locate the element above the trap’s condensate leg for accurate temperature measurement. If possible, use trap bypass valves for efficient air purging at the process low point to minimize the mixing of steam and air.
5. Use Monitored High Point Air Bleeds
The importance of complete air elimination from the processing system cannot be overstated. To assist in the complete replacement of air with saturated steam, use monitored high-point air bleeds to push high air pockets out of the system. High-point air bleeds are recommended on large filter housing, process vessel vent and overlay gas supply lines.
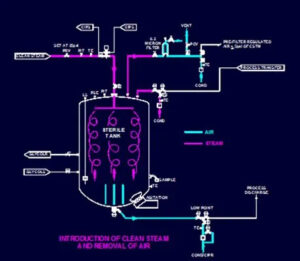
6. Avoid Parallel Steam Paths
Whenever possible, avoid parallel steam paths within the processing system sharing a common steam trap. As pressure differences occur during air removal stages, parallel lines pocket air, which prevents proper steam exposure, and results in sterility failures.
7. Plan Steam Flow and Process Flow in the Same Direction
Plan the SIP operation to have steam flow in the same direction as the process flow to take advantage of the existing process piping pitch and support for process drainability. Plan the piping system to avoid hoses, dead-ends, and non-drainable low points that can create condensate pools or air pockets. These pools and air pockets can provide an insulating effect on the targeted microorganisms. Prompt and effective condensate elimination is critical to the SIP Operation’s success.
8. Include a Time Delay
After process air has been fully removed and the system charged with saturated steam, a time delay is recommended for all system components to achieve the desired set point temperature. To achieve sterile conditions, universally expose the system to the FDA Center for Biologics Evaluation and Research (CBER) 21 CFR Guideline (Section 600.11) Time – Temperature Relationship of 121.5°C for 20 minutes. Consider adding an additional 1.5°C to the SIP set point to cover temperature element accuracy concerns. The 20-minute thermal exposure must be continuous, without the temperature dropping below the set point.
9. Active Steam Flow During SIP Hold
Providing a means of active steam flow to the process as the SIP timer counts down can help ensure the required thermal treatment has been achieved. This steam “activity” can be achieved by positioning the process vessel vent pressure control valve slightly open, or through the use of orificed steam traps or bypass valves.
10. Protect Sterile Process Boundary
After the desired exposure to saturated steam has been achieved for a complete thermal bio-kill, the sterile process boundary must be protected as the condensate is drained from the system. The equipment is cooled to prepare for sterile process operations.
Once the sterile boundary has been created, the question is often raised “How does one protect and maintain a sterile process boundary?” The use of overlapping sterile boundaries, active or passive air overlays, and steam blocks can be employed with great success.
 About the author: Mike Byron is Haskell’s lead process engineer and has been with the company since 1993. He is intensively involved in Process/CIP/SIP system design for food and pharmaceutical manufacturing facilities. His diverse process engineering knowledge includes automated sterile/aseptic processing systems for cell culture, harvest, purification and sterile filling operations. He earned bachelor’s degrees in agricultural engineering and biochemistry from Purdue University.
About the author: Mike Byron is Haskell’s lead process engineer and has been with the company since 1993. He is intensively involved in Process/CIP/SIP system design for food and pharmaceutical manufacturing facilities. His diverse process engineering knowledge includes automated sterile/aseptic processing systems for cell culture, harvest, purification and sterile filling operations. He earned bachelor’s degrees in agricultural engineering and biochemistry from Purdue University.
Contact our Process Engineering team to learn more about maintaining sterile conditions or to discuss your specific facility.



 About the author:
About the author: